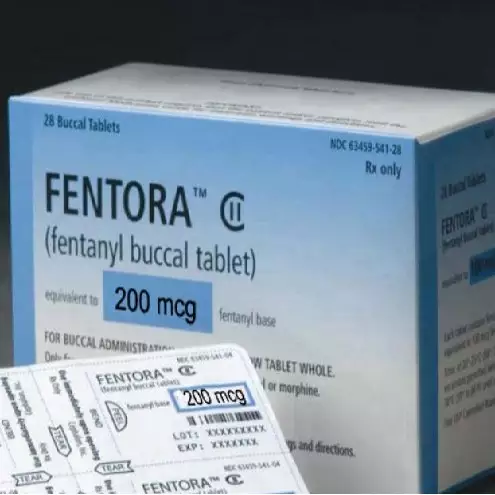
Authorized Generic version of Fentora has been approved.
An Authorized Generic is a prescription drug that is produced by a brand company under a New Drug Application (NDA) and marketed as a generic under a private label. It is identical to the branded product in appearance and has exactly the same inactive ingredients.
List of authorized generic versions:
- Fentanyl Citrate BUCCAL; SUBLINGUAL TABLET 100 mcg
Mayne Pharma
NDC Code: 518620634 - Fentanyl Citrate BUCCAL; SUBLINGUAL TABLET 200 mcg
Mayne Pharma
NDC Code: 518620635 - Fentanyl Citrate BUCCAL; SUBLINGUAL TABLET 400 mcg
Mayne Pharma
NDC Code: 518620636 - Fentanyl Citrate BUCCAL; SUBLINGUAL TABLET 600 mcg
Mayne Pharma
NDC Code: 518620637 - Fentanyl Citrate BUCCAL; SUBLINGUAL TABLET 800 mcg
Mayne Pharma
NDC Code: 518620638






Reviews
There are no reviews yet.